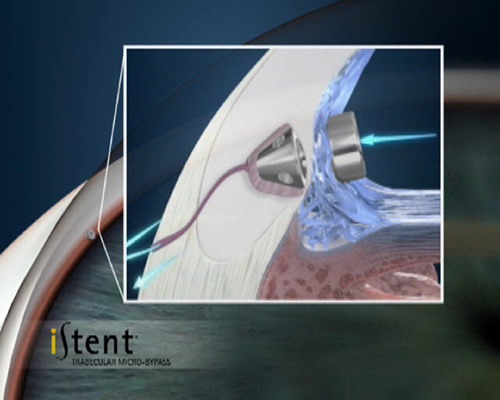
 Glaucoma treatment involves the lowering of intraocular pressure. This pressure is created by the ciliary body (an area in the front of the eye behind the Iris) producing a fluid called aqueous humor that leaves the eye against some resistance across some specialised tissue in the angle between the iris and cornea. Treatment usually commences with eyedrops but sometimes Laser or Surgical interventions are required.
Glaucoma treatment involves the lowering of intraocular pressure. This pressure is created by the ciliary body (an area in the front of the eye behind the Iris) producing a fluid called aqueous humor that leaves the eye against some resistance across some specialised tissue in the angle between the iris and cornea. Treatment usually commences with eyedrops but sometimes Laser or Surgical interventions are required.
What is MIGS?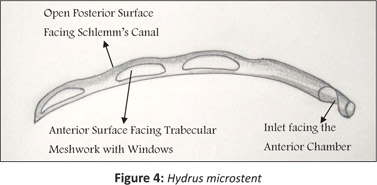
The term MIGS refers to Micro Invasive Glaucoma Surgery.
Over the past several years alternatives to the standard forms of glaucoma surgery (Trabeculectomy & Tube/Shunt procedures) have been developed. MIGS involves the insertion of one of several different styles of devices into the front part of the eye with the aim of lowering intra ocular pressure. While Trabeculectomy & Tube procedures remain important in the treatment of glaucoma they are complex procedures requiring significant operative time and postoperative follow up to maximise success. MIGS procedures are simpler with generally little postoperative follow up and are often combined with cataract surgery.
 Types of MIGS
Types of MIGS
Several types of MIGS devices exist but all have a similar principle, to allow the aqueous fluid easier passage out of the eye.
- Trabecular shunts: Glaukos Istent & Istent Inject; Ivantus Hydrus.
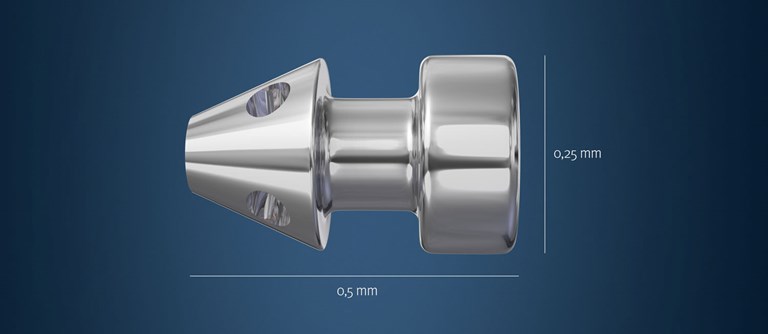
These are the currently most commonly used of the MIGS devices. The Istent is presently only registered for implantation in conjunction with cataract surgery. Via a small corneal incision a preloaded device is inserted into the eye and a tiny titanium shunt is accurately positioned into the trabecular meshwork between the iris and cornea.
The Hydrus device may be inserted as a standalone procedure or combined with cataract surgery. A curved scaffold is inserted into the trabecular meshwork in a similar way to the Istent.
- Suprachoroidal shunts: Cypass device
This device is a larger drainage tube that is again placed in the area between iris and cornea but drains the aqueous to an area between the vascular choroid layer and the outer fibrous wall of the eye, the sclera.
- Subconjunctival devices : Xen device
Yet to be registered in Australia this MIGS device is in use in the USA and United Kingdom. It consists of a fine microfilament inserted via a needle through the trabecular meshwork then exiting in the subconjunctival space. Aqueous fluid can then track down this microfilament out of the eye thus mimicking the trabeculectomy and tube/shunt operations but without the more extensive surgery.
 Who is suitable for MIGS?
Who is suitable for MIGS?
Not all patients with glaucoma are suitable for MIGS. Generally it applies to open angle glaucoma and may not be suitable for patients with advanced disease. Your ophthalmologist will be able to advise you.
The Procedure
This form of surgery is often combined with cataract surgery and adds little time and risk to the cataract surgery. It is typically done under local anaesthetic and involves the placement of the small shunt into the trabecular meshwork in the angle between the iris and cornea. Post operatively the eye is treated with antibiotic and anti- inflammatory eye drops. The risks associated with the procedure are very low. There can be some mild bleeding which is usually self- limited and mild intraocular inflammation. As with any intraocular procedure there is a very small risk of infection.
 Results
Results
The largest amount of literature on MIGS devices covers trabecular stenting devices namely the Glaukos Istent. Most studies have shown a decrease in intraocular pressure compared with cataract surgery alone. They also generally report a decrease in the required number of eye drops. However there is some individuality in response with some patients under responding and the duration of effect of this device may not be indefinite but it seems to last in excess of two years from published reports.
